How ReFlow™ Helped Get Zachary’s Hydrocephalus Under Control and Travel Across the Globe
 Even before Zachary was born nearly 11 years ago, his parents Sherry and Jason knew that he was going to be a special boy – and a wonderful sibling to a twin sister! Zachary was born with spina bifida and hydrocephalus, both of which were diagnosed while in utero.
Even before Zachary was born nearly 11 years ago, his parents Sherry and Jason knew that he was going to be a special boy – and a wonderful sibling to a twin sister! Zachary was born with spina bifida and hydrocephalus, both of which were diagnosed while in utero.
As with many children in the hydrocephalus community, most parents have never heard of the condition, nor understand how it could impact their child’s life, as well as impact their own emotional health with concern, worry, and stress.
Upon his birth at 35 weeks, Zachary immediately received a shunt device to ensure the cerebrospinal fluid in his brain drained to avoid any swelling, which could potentially stunt normal and expected development. However, like many living with hydrocephalus, reoccurring blockages created near-continuous fears and concerns, as each episode required clinical interventions and hospitalizations.
This stressful journey continued until 2019 when Zachary had the ReFlow device implanted by Dr. Michael Muhonen, a neurosurgeon at Orange County Children’s Hospital, enabling a more proactive approach to ensure properly performing shunts. Dr. Muhonen directed Sherry and Jason to use the ReFlow device to ‘flush’ Zachary’s shunt each day as part of their goodnight routine or whenever Zachary was beginning to experience a headache. With the simple push of a button (the ReFlow Flusher), Zachary’s parents were able to participate in managing his hydrocephalus and found themselves less stressed about the unexpected and unpredictable – all in under 30 seconds.
 “Now at 10 years old, Zachary is living his life without as many concerns related to his hydrocephalus. Before the ReFlow being implanted, taking adventures was never a reality, as unexpected needs could arise at a moment’s notice. However, this past November Zachary and Jason were able to travel to Egypt to support important mission work by distributing wheelchairs – a memorable experience that most likely would have never happened without the ReFlow. Our family, who is also enjoying national travel and fun everyday activities such as biking, is thrilled to live our lives with a greater sense of confidence that Zachary’s hydrocephalus is under control,” Sherry shared.
“Now at 10 years old, Zachary is living his life without as many concerns related to his hydrocephalus. Before the ReFlow being implanted, taking adventures was never a reality, as unexpected needs could arise at a moment’s notice. However, this past November Zachary and Jason were able to travel to Egypt to support important mission work by distributing wheelchairs – a memorable experience that most likely would have never happened without the ReFlow. Our family, who is also enjoying national travel and fun everyday activities such as biking, is thrilled to live our lives with a greater sense of confidence that Zachary’s hydrocephalus is under control,” Sherry shared.
The ReFlow™ System Mini and ReFlow™ Mini Flusher are FDA cleared for the following indication:
The ReFlow System Mini and the ReFlow Mini Flusher, used as components of a shunt system, are for use in the treatment of patients with hydrocephalus or conditions where draining or shunting of cerebrospinal fluid (CSF) is medically indicated. The miniaturized ReFlow Mini Flusher may be used by a qualified clinician as a tool to facilitate a noninvasive retrograde fluid flush of the shunt ventricular catheter to unblock inlet holes to restore, increase, or maintain CSF flow. When used with the ReFlow Ventricular Catheter, the flush can also open the ReFlow Ventricular Catheter’s relief membrane to restore, increase, or maintain CSF flow. The ReFlow System Mini components are not intended to change the diagnosis, treatment, or follow-up of patients with proximal catheter occlusions. Under the care, direction, and instruction of the treating physician, the ReFlow Mini Flusher may be used as directed for noninvasive flushing by a trained healthcare professional in-clinic or by a trained caregiver or adult patient in a non-clinical environment.

Anuncia Medical Inc. plans to work closely with the medical community and the FDA to conduct larger scale studies to further evaluate use of the ReFlow device to maintain flow.




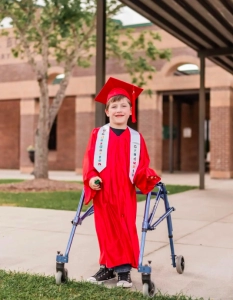 After struggling to conceive a child for what felt like ages, Miranda of Moncks County, South Carolina finally fell pregnant – a miracle that quickly turned into horror when she delivered at 24 weeks in late 2015. At just 1 pound and 12 ounces, her son, Sutton, arrived with life-threatening health issues, including hydrocephalus – a neurological disorder caused by an abnormal buildup of cerebrospinal fluid in the brain that
After struggling to conceive a child for what felt like ages, Miranda of Moncks County, South Carolina finally fell pregnant – a miracle that quickly turned into horror when she delivered at 24 weeks in late 2015. At just 1 pound and 12 ounces, her son, Sutton, arrived with life-threatening health issues, including hydrocephalus – a neurological disorder caused by an abnormal buildup of cerebrospinal fluid in the brain that 


